|
Serology Testing
Types of Specimen Collection
 Currently there are point of care and high complexity serological tests with emergency use authorizations. As with diagnostic testing, pharmacies can partner with labs* to perform high complexity tests in several ways to ensure patients have access to serology testing. Currently there are point of care and high complexity serological tests with emergency use authorizations. As with diagnostic testing, pharmacies can partner with labs* to perform high complexity tests in several ways to ensure patients have access to serology testing.
Specimens for antibody tests differ from diagnostic testing. Most antibody test specimens are blood samples. Another specimen type that may be used in serological testing is saliva.
Antibody tests are performed using blood samples which can be collected through a finger stick or a blood draw. The common blood collection technique that applies to pharmacy are finger stick collections. Proper technique should be used for collecting blood samples. See the CDC Interim Guidelines for Collecting and Handling of Clinical Specimens for COVID-19 Testing for a review of the proper technique:
*see potential lab partners section
Potential Lab Partners and Point of Care Platforms
 Under Section 6001(a) of the FFCRA, as amended by section 3201 of the CARES Act, insurance plans are required to provide coverage for serological testing for COVID-19 that has been ordered by a provider for the duration of the public health emergency. Based on HHS guidance, pharmacists are also permitted to order tests even if the test is not performed onsite at the pharmacy. Pharmacies can administer the approved point of care tests in the pharmacies or act as a specimen collection site for tests that require higher complexity CLIA certifications. Patients can also be directed to their provider who can order an antibody test through a clinical laboratory. Serology testing is currently prescription-based, and pharmacists are permitted to determine if antibody testing is appropriate for the patient and order the test. There are some laboratories, such as Quest Diagnostics, offering antibody testing directly to the public by using their own independent physician to oversee and order testing. This option requires the patient to pay out of pocket for the test directly rather than going through their insurance. Under Section 6001(a) of the FFCRA, as amended by section 3201 of the CARES Act, insurance plans are required to provide coverage for serological testing for COVID-19 that has been ordered by a provider for the duration of the public health emergency. Based on HHS guidance, pharmacists are also permitted to order tests even if the test is not performed onsite at the pharmacy. Pharmacies can administer the approved point of care tests in the pharmacies or act as a specimen collection site for tests that require higher complexity CLIA certifications. Patients can also be directed to their provider who can order an antibody test through a clinical laboratory. Serology testing is currently prescription-based, and pharmacists are permitted to determine if antibody testing is appropriate for the patient and order the test. There are some laboratories, such as Quest Diagnostics, offering antibody testing directly to the public by using their own independent physician to oversee and order testing. This option requires the patient to pay out of pocket for the test directly rather than going through their insurance.
As mentioned above, there are possibilities for pharmacies to serve as sites for specimen collection when partnering with laboratories and local health departments. These other entities would be responsible for actually performing the test. There are also multiple point of care rapid tests available for administration in the pharmacy. Similar to diagnostic tests, serological tests that can be performed at the point of care will be noted with a “W” indicating CLIA waived. Antibody tests that have been granted Emergency Use Authorizations are listed here:
For the most up to date information on serology testing, visit the following FDA websites:
(https://www.cms.gov/files/document/FFCRA-Part-42-FAQs.pdf)
Recommendations for Supplies, PPE, and Equipment
 Similar to diagnostic testing, the required PPE includes, but is not limited to face shields and masks. Although serology tests are not testing for an active infection, proper precautions should be followed to reduce potential exposures to COVID-19. Since blood samples are being collected, additional safety equipment that is important for collection includes gloves and sharps containers. Similar to diagnostic testing, the required PPE includes, but is not limited to face shields and masks. Although serology tests are not testing for an active infection, proper precautions should be followed to reduce potential exposures to COVID-19. Since blood samples are being collected, additional safety equipment that is important for collection includes gloves and sharps containers.
Pharmacies should follow all standard preventive measures outlined by the CDC and NCDHHS. Special considerations may be implemented for pharmacies that are also COVID-19 viral testing sites. Additionally, the CDC generally recommends source control and physical distancing for healthcare workers. Up to date recommendations for PPE and infection control can be found using the CDC Infection Control Guidance for Healthcare Professionals about Coronavirus (COVID-19):
Safety Protocol
 Patients should call their doctor and discuss whether or not an antibody test is appropriate for them. Then, depending on the laboratory that provides the testing, patients will be directed to go either to their doctor’s office, pharmacy, or a satellite laboratory location to collect the blood sample required for the antibody test. If patients are feeling ill or have a fever on the day they are scheduled to give a blood sample, they should stay at home and talk to their doctor about rescheduling their appointment. Patients should expect their results in as little as 15 minutes or up to 4 days, depending on the location and type of test used. Patients should call their doctor and discuss whether or not an antibody test is appropriate for them. Then, depending on the laboratory that provides the testing, patients will be directed to go either to their doctor’s office, pharmacy, or a satellite laboratory location to collect the blood sample required for the antibody test. If patients are feeling ill or have a fever on the day they are scheduled to give a blood sample, they should stay at home and talk to their doctor about rescheduling their appointment. Patients should expect their results in as little as 15 minutes or up to 4 days, depending on the location and type of test used.
Patient Checklist
 Prior to entering a collection site, pharmacy, or doctor’s office, patients should be screened for symptoms of an active COVID-19 infection. Providers should ask if the patient has had any exposure to COVID-19 in the last two weeks and check if they are experiencing any flu-like symptoms. Patients should be instructed to adhere to current infection control guidelines while inside the collection site or doctor’s office. Prior to entering a collection site, pharmacy, or doctor’s office, patients should be screened for symptoms of an active COVID-19 infection. Providers should ask if the patient has had any exposure to COVID-19 in the last two weeks and check if they are experiencing any flu-like symptoms. Patients should be instructed to adhere to current infection control guidelines while inside the collection site or doctor’s office.
(https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html)
Who Should be Tested for COVID-19 Antibodies?
 The CDC does not recommend using antibody testing for the diagnosis of current acute infection. Antibodies developed from an infection may take around two weeks to develop. Patients who are recovering from a recent COVID-19 infection should wait some time before considering an antibody test. Potential candidates for an antibody test include those who may have been exposed and do not have any current symptoms or those who were exposed and recovered. The CDC does not recommend using antibody testing for the diagnosis of current acute infection. Antibodies developed from an infection may take around two weeks to develop. Patients who are recovering from a recent COVID-19 infection should wait some time before considering an antibody test. Potential candidates for an antibody test include those who may have been exposed and do not have any current symptoms or those who were exposed and recovered.
(https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests-guidelines.html)
(https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests.html)
Interpreting Results
Antibody tests should not be used for the diagnosis of a current infection. It is also not recommended to use antibody testing as a method for determining immunity to COVID-19. Antibody tests can be used to help determine a timeline of infection when results are used alongside a diagnostic test, as well as establish exposure statistics for the population. The following chart can be used to help interpret the results of antibody and viral tests when used concurrently:
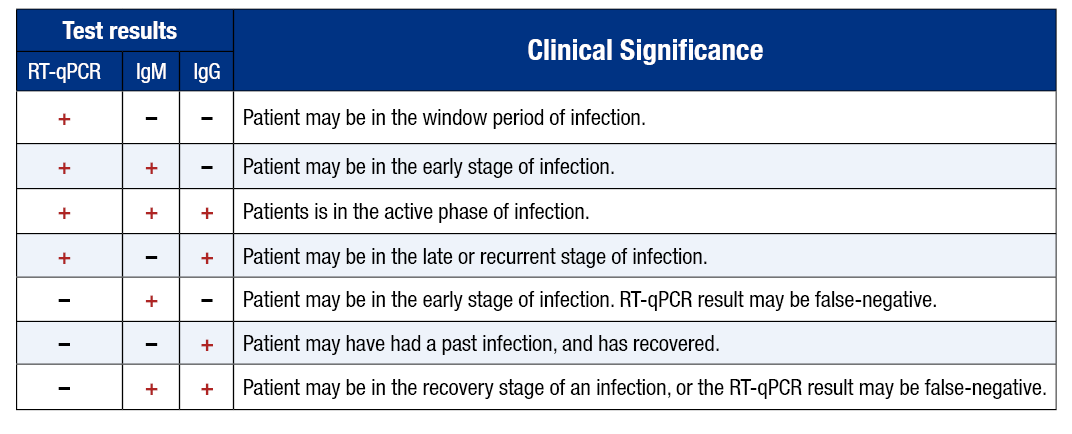
To KK, Tsang OT, Leung WS, Tam AR, Wu TC, Lung DC et al. (2020). Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020 Mar 23. pii: S1473-3099(20)30196-1
When an antibody test alone is administered, results can be interpreted as follows:
-
A positive test result indicates the patient has likely been exposed to COVID-19 and developed antibodies from a past infection or vaccination. It is also possible that the patient developed these antibodies in response to an infection by other viruses from the same coronavirus family, and this would indicate a false positive for COVID-19. It is not recommended to use positive antibody test results as a method for determining immunity to COVID-19. As a result, it is important to educate patients on protective guidelines to prevent being infected with the virus again.
-
If the patient tests negative for antibodies, they likely have not had COVID-19 in the past or they are in the early stages of infection. It can take several weeks for antibodies to reach a detectable level, and it is also possible that some people may not develop antibodies at all if they have been exposed. Some antibody tests can detect antibodies from vaccination, but others cannot. Therefore, a test that is negative for antibodies does not necessarily mean that a patient has never received a COVID-19 vaccination.
(https://www.cdc.gov/coronavirus/2019-ncov/testing/serology-overview.html)
Reporting Results
 Reporting requirements for antibody testing are rapidly changing as the volume of testing increases and more information about COVID-19 comes out. Antibody test results that are reported are utilized by the CDC to establish patterns and exposure within populations, geographic locations, and demographics. Some states have reported diagnostic and antibody test results together. However, since these types of tests provide different kinds of results, the numbers that have been reported may not be an accurate representation of current and previous COVID-19 cases. Many states are now requiring diagnostic and antibody test results to be reported separately. NCDHHS requires clinicians and laboratories to immediately report suspected or confirmed COVID-19 cases to state or local health departments. Aside from reporting to health departments, the North Carolina Board of Pharmacy (NCBOP) requires COVID-19 test results to be reported to the patient and the patient’s health care provider. These mandatory reporting requirements are not currently in place for antibody testing. Reporting requirements for antibody testing are rapidly changing as the volume of testing increases and more information about COVID-19 comes out. Antibody test results that are reported are utilized by the CDC to establish patterns and exposure within populations, geographic locations, and demographics. Some states have reported diagnostic and antibody test results together. However, since these types of tests provide different kinds of results, the numbers that have been reported may not be an accurate representation of current and previous COVID-19 cases. Many states are now requiring diagnostic and antibody test results to be reported separately. NCDHHS requires clinicians and laboratories to immediately report suspected or confirmed COVID-19 cases to state or local health departments. Aside from reporting to health departments, the North Carolina Board of Pharmacy (NCBOP) requires COVID-19 test results to be reported to the patient and the patient’s health care provider. These mandatory reporting requirements are not currently in place for antibody testing.
(https://www.cdc.gov/coronavirus/2019-ncov/lab/reporting-lab-data.html)
(https://covid19.ncdhhs.gov/dashboard/about-data)
(https://covid19.ncdhhs.gov/media/456/download)
Documentation
 Documentation for antibody testing will be similar to documentation for diagnostic testing, please refer back to that section. The location where the patient’s blood sample was taken will be responsible for documenting information for the antibody test, as well as the results received. Documentation for antibody testing will be similar to documentation for diagnostic testing, please refer back to that section. The location where the patient’s blood sample was taken will be responsible for documenting information for the antibody test, as well as the results received.
Patient Information and Education
What the Test Does
 An antibody test can determine whether a patient has been previously infected with COVID-19 or it can potentially detect previous vaccination against COVID-19. This type of test does not indicate if the patient has a current infection. It is also not recommended to use antibody testing as a method for determining immunity to COVID-19. However, widespread antibody testing is currently being conducted to better understand and track COVID-19 infections across time, locations, and different populations. An antibody test can determine whether a patient has been previously infected with COVID-19 or it can potentially detect previous vaccination against COVID-19. This type of test does not indicate if the patient has a current infection. It is also not recommended to use antibody testing as a method for determining immunity to COVID-19. However, widespread antibody testing is currently being conducted to better understand and track COVID-19 infections across time, locations, and different populations.
When the Results Might Be Ready
Results may be available in as little as 15 minutes for a rapid test or may take several days to return. The timeline will depend on the test used and the location where testing takes place.
Interpreting Results
A positive test result indicates the patient has likely been exposed to COVID-19 and developed antibodies from a past infection or vaccination. It is also possible that the patient developed these antibodies in response to an infection by other viruses from the same coronavirus family, and this would indicate a false positive for COVID-19. It is not recommended to use positive antibody test results as a method for determining immunity to COVID-19. As a result, it is important to educate patients on protective guidelines to prevent being infected with the virus again. If the patient tests negative for antibodies, they likely have not had COVID-19 in the past or they are in the early stages of infection. It can take several weeks for antibodies to reach a detectable level, and it is also possible that some people may not develop antibodies at all if they have been exposed. Some antibody tests can detect antibodies from vaccination, but others cannot. Therefore, a test that is negative for antibodies does not necessarily mean that a patient has never received a COVID-19 vaccination. It is important to emphasize to the patient that antibody tests can not be used to diagnose a current infection and that at this time they do not indicate immunity to future infections.
(https://www.cdc.gov/coronavirus/2019-ncov/testing/serology-overview.html)
What Actions to Take After Getting Results
If the patient tests positive for antibodies but has no symptoms, they do not likely have a current infection and additional follow-up is not required. If the patient tests positive for antibodies and also has symptoms of COVID-19, they should consider obtaining a diagnostic test to determine whether a current infection is present. Patients should report positive results to their healthcare provider. If the antibody test is negative, the patient should still consider diagnostic testing if they have symptoms or develop them in the future. Regardless of the results, the patient should continue to take preventive measures such as frequent hand washing, cleaning/disinfecting, avoiding close contact, wearing cloth face coverings in public, and self-monitoring for symptoms of potential infection.
(https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/prevention.html)(https://www.cdc.gov/coronavirus/2019-ncov/downloads/10Things.pdf)
|



